TERBIUM-161
The first commercial Terbium-161 product !
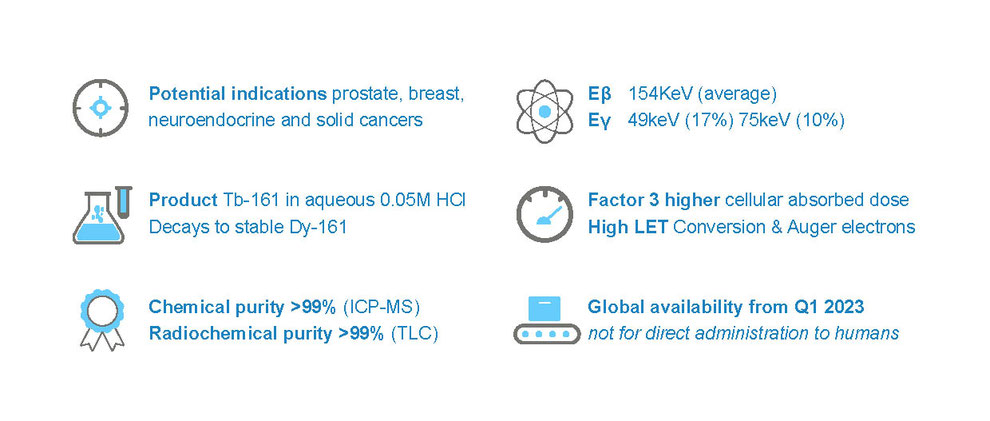
Terbium-161 produced at the highest radiochemical grade under non-GMP conditions, available in 0.05M HCl Solution. Current intended use: not for direct administration to humans.
Carrier-free lanthanide (NCA) Terbium-161 is produced by neutron activation of the highly enriched Gadolinium-160 using small and medium research reactors. As a promising radionuclide for Radioligand Therapy, Terbium-161 exhibits comparable chemical characteristics to known radiolanthanides allowing utilization of current radiolabeling techniques.
From a clinical perspective, early research suggests improved anti-tumor capabilities, especially for (micro)metastasized cancers, owing to an increase in emission of Auger and conversion electrons when using terbium-161 compared to other known radiolanthanides.

Radioligand Therapy
Using PSMA and SST analogues, Terbium-161 has shown excellent bioequivalence presenting a biodistribution comparable to the currently used radiolanthanides. Additionally, the 16-fold increase in Auger and conversion electrons is expected to improve the cellular absorbed dose up to 3-fold compared to currently used radiolanthanides, while allowing for treatment of both primary and undetectable (micro) metastasis, improving the overall disease control.
Imaging
The emitted gamma radiation can be used for whole-body planar as well as for SPECT/CT when energy window is centered at 74.6KeV.
Allowing optimization of the SPECT reconstruction parameters, Terbium-161 may provide for higher spatial resolution SPECT imaging, leading to the detection of smaller lesions that provides input for a further optimized treatment plan.
Radiation Safety
Terbium-161, compared to current standard radiolanthanides, provides for an immediate decrease in dose rate allowing a significant improvement in general radiation safety. This optimization of radiation safety may extend current treatment room capacity for Radioligand Therapy. The non-carrier added (NCA) product allows optimal control of radioactive waste flow with reduced decay time in local storage.

Minervum 7070
4817 ZK Breda
The Netherlands

